EMPower your patients with impactful results
EMPAVELI was superior to a C5 inhibitor (C5i) (eculizumab) in improving Hb1,2
3.84 g/dL difference with EMPAVELI compared to eculizumab at Week 161,*,†
(P<0.0001) (95% Cl, 2.33-5.34)
Hb mean values observed (±SE, ITT, not censored for transfusions) from baseline to Week 481,3-5,†
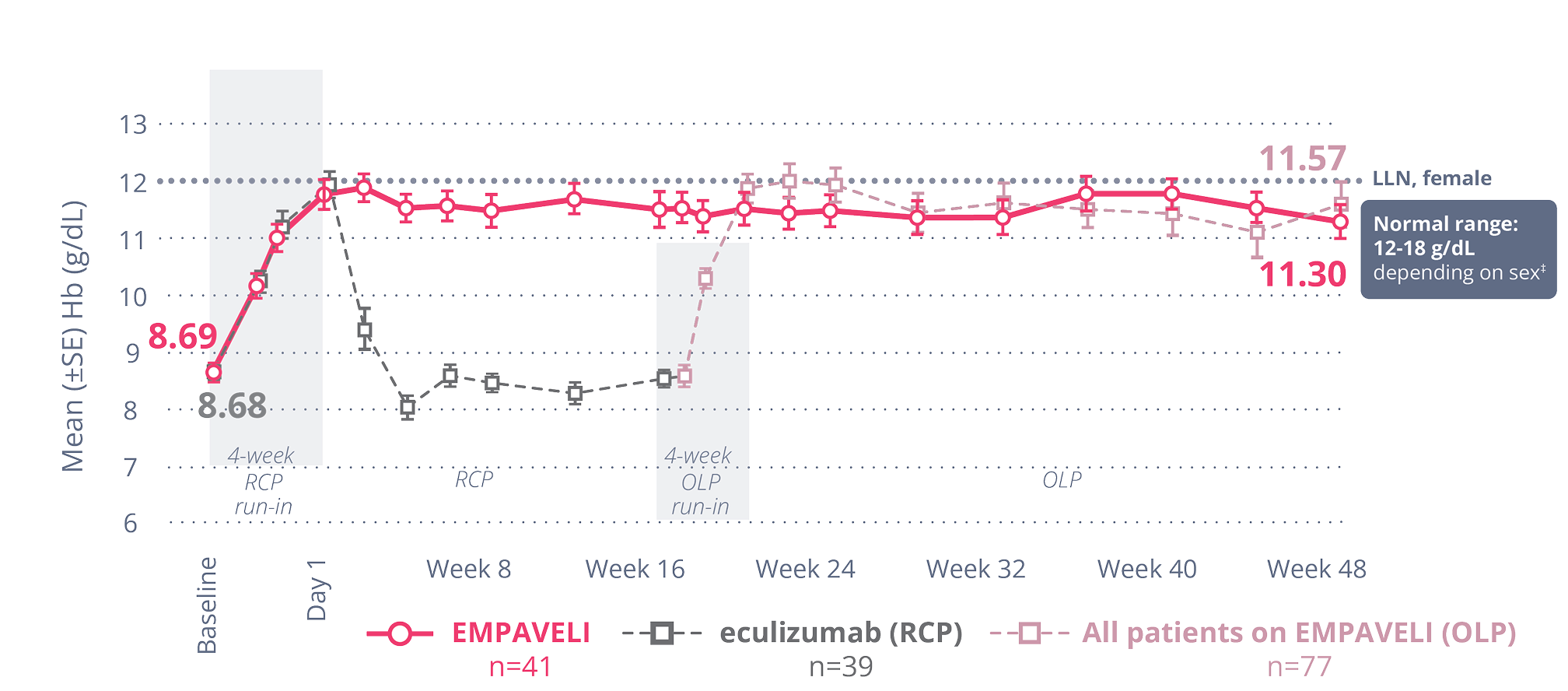
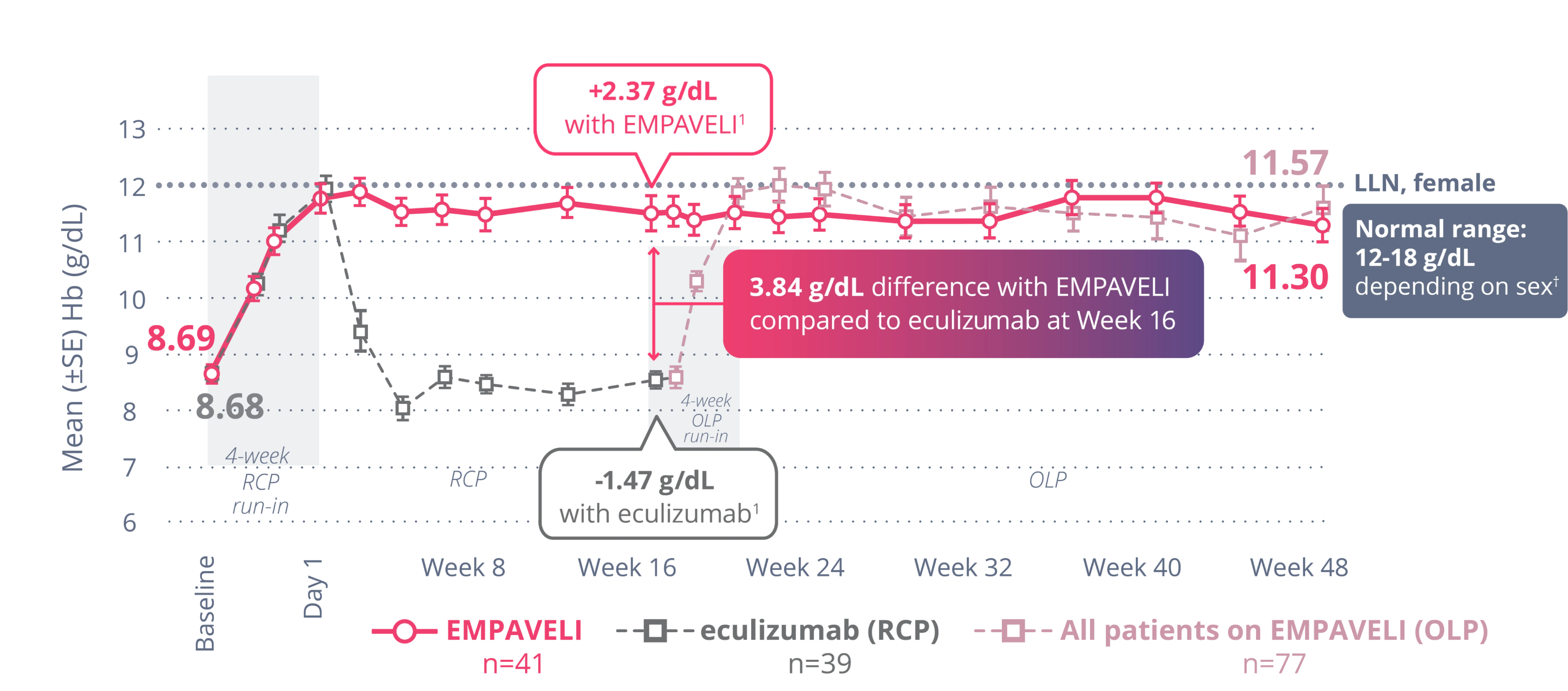
†Primary analysis of the PEGASUS data is censored for transfusion, whereas OLP observed data do not censor posttransfusion data. Transfusions could confound the results, so data after the first transfusion for all patients were not included in the primary analysis. Posttransfusion data were omitted once they had a transfusion and their data were modeled out for the remainder of the 16-week RCP.2
*Treatment effect estimates from a mixed-effects model are shown. The mixed-effects model contained the categorical effects of treatment, visit, treatment by visit interaction, and stratification factors (transfusion history and platelet count at screening), and the continuous covariate of baseline value.1
‡Normal range of Hb: 12 to 16 g/dL for females, 13.6 to 18 g/dL for males.3

Individual patient experiences may vary.
EMPAVELI kept most patients transfusion free through Week 161
Transfusion avoidance at Week 16 met noninferiority1,§
Remaining transfusion free may have significant clinical benefits for patients.6
§Noninferiority is a type of analysis that tests whether a new treatment is not worse than a comparator treatment by more than a specified margin.3
||Sixty-three percent adjusted treatment difference between EMPAVELI and eculizumab (95% CI, 48%-77%). Difference in percentages and 95% CI were based on the stratified Miettinen Nurminen method.5

Individual patient experiences may vary.
Noninferiority was met for the change from baseline in ARC level at Week 16.3
EMPAVELI patients saw a decrease in ARC of -136 x 109 cells/L from baseline. Eculizumab patients saw an increase in ARC of 28 x 109 cells/L.3,¶
randomized controlled period (ITT set)5
¶Difference of mean values was -164 between EMPAVELI (SE, 6.5; n=41) and eculizumab (SE, 11.9; n=39) (95% CI, -189.9 to -137.3).3
Noninferiority was not met for the change from baseline in LDH level at Week 16.3
The data are descriptive in nature and are for observation only. No formal comparisons can be drawn between the 2 arms.
The data are descriptive in nature and are for observation only. No formal comparisons can be drawn between the 2 arms.
EMPAVELI patients saw an adjusted mean change in LDH level of -15 U/L from baseline. Eculizumab patients saw an adjusted mean change in LDH level of -10 U/L from baseline.3
randomized controlled period (ITT set)5,#
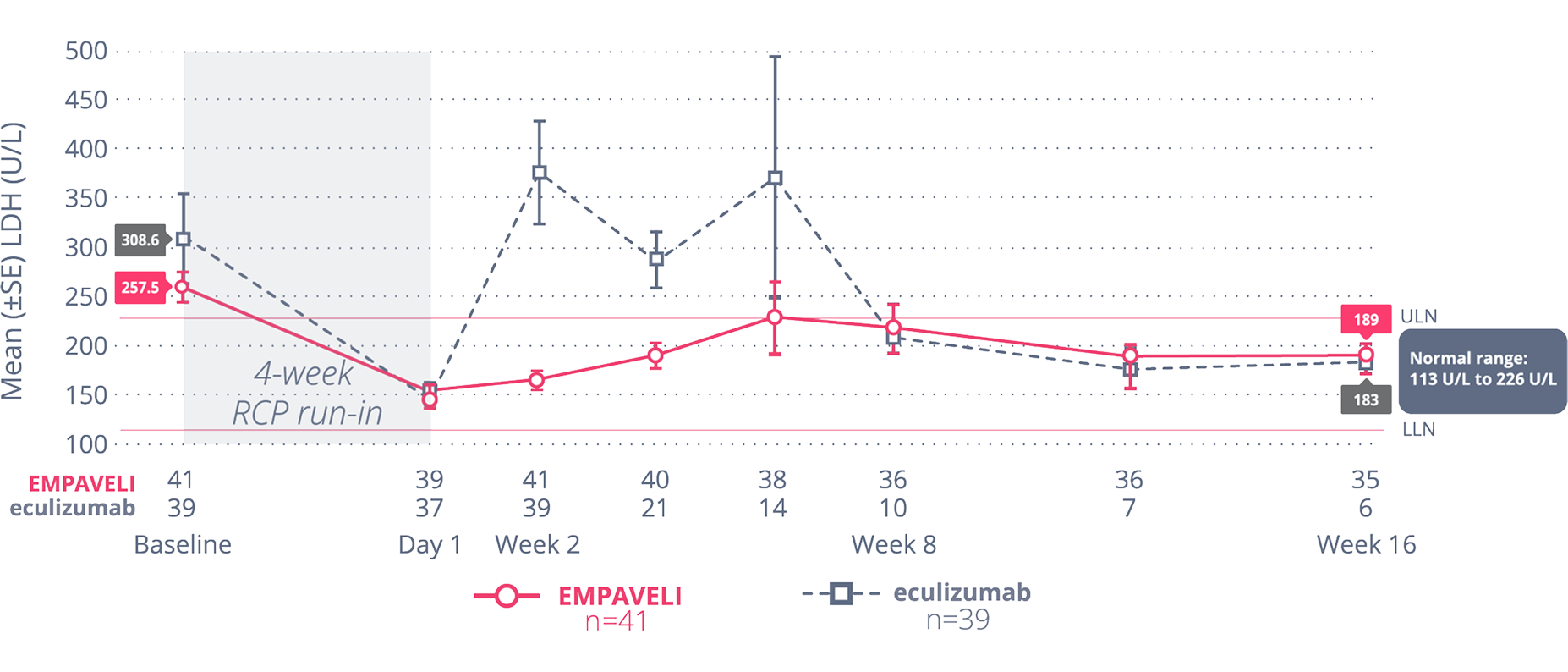
#Primary analysis of the PEGASUS data is censored for transfusion. Transfusions could confound the results, so data after the first transfusion for all patients were not included in the primary analysis in order to truly assess the effect of each drug. Data posttransfusion were omitted once patients had a transfusion and their data were modeled out for the remainder of the 16-week RCP.3,5
Change from baseline in FACIT-Fatigue score was not tested for noninferiority, due to
hierarchical testing.3
The data are descriptive in nature and are for observation only. No formal comparisons can be drawn between the 2 arms.
The data are descriptive in nature and are for observation only. No formal comparisons can be drawn between the 2 arms.
A ≥5-point difference is generally considered clinically meaningful.8
**Scores on the FACIT-Fatigue scale range from 0 to 52, with higher scores indicating less fatigue.
††Primary analysis of the PEGASUS data is censored for transfusion. Transfusions could confound the results, so data after the first transfusion for all patients were not included in the primary analysis in order to truly assess the effect of each drug. Data posttransfusion were omitted once patients had a transfusion and their data were modeled out for the remainder of the 16-week RCP.3,5
Patients with greater improvements in hemoglobin showed a larger reduction in fatigue.8
hemoglobin at Week 16 (n=72)5,8
An Hb increase of ≥1 g/dL had an average fatigue improvement of about 10 points.8
- Higher Hb levels indicated lower levels of fatigue8
- These post hoc analyses included patients receiving EMPAVELI and eculizumab8
72% of patients on EMPAVELI achieved FACIT-Fatigue scores at the level of the general population vs 21% on eculizumab8
‡‡FACIT-Fatigue normalization was achieved when patient scores reached the general population mean of ≥43.6. FACIT-Fatigue normalization was achieved by 72% (n=23/32) of EMPAVELI patients and 21% (n=6/29) of eculizumab patients.9 Scores on the FACIT-Fatigue scale range from 0 to 52, with higher scores indicating less fatigue. A ≥5-point difference is generally considered clinically meaningful.8
EMPAVELI normalized Hb and LDH levels in C5i-experienced (eculizumab) patients1-3,5
The data from these secondary analyses are descriptive in nature and collected for observation only. No formal comparisons can be drawn between the 2 arms.
More than 1/3 of patients achieved Hb normalization with EMPAVELI at Week 163,§§
normalization
was achieved in 34% of patients taking EMPAVELI (n=41)
The majority of patients achieved LDH normalization with EMPAVELI at Week 163,||||
normalization
was achieved in 71% of patients taking EMPAVELI (n=41)
Both analyses were censored for transfusions.5
§§Hb normalization is defined as an Hb level at or above the lower limit of the gender-specific normal range, which is ≥12 g/dL to 16 g/dL for females and ≥13.6 g/dL to 18 g/dL for males.3,5
||||LDH normalization is defined as an LDH level at or below the upper limit of normal range, which is 113 U/L to 226 U/L.5
Most patients on EMPAVELI achieved Hb stabilization at Week 261
Hb stabilization
was achieved in 85.7% of patients
taking EMPAVELI (n=35)¶¶
at Week 261
(P<0.0001) (95% Cl, 57%-89%)
In PRINCE, Hb stabilization was defined as avoidance of a >1 g/dL decrease in Hb levels from baseline in the absence of transfusions through Week 26 (yes/no)1,5
¶¶Patients who received a transfusion, escaped from control arm to the EMPAVELI treatment group, withdrew from trial, or were lost to follow-up were categorized as nonresponders. Cochran-Mantel-Haenszel test stratified by number of RBC transfusions within 12 months prior to screening (<4, ≥4).1,10
##Eleven of 18 patients randomized to the control transitioned to crossover therapy with EMPAVELI due to a decreased Hb level ≥2 g/dL below baseline.1
EMPAVELI achieved rapid and sustained LDH reductions
through Week 261,10
EMPAVELI reduced LDH to normal levels by Week 2, an effect that was sustained
through Week 261,5,10
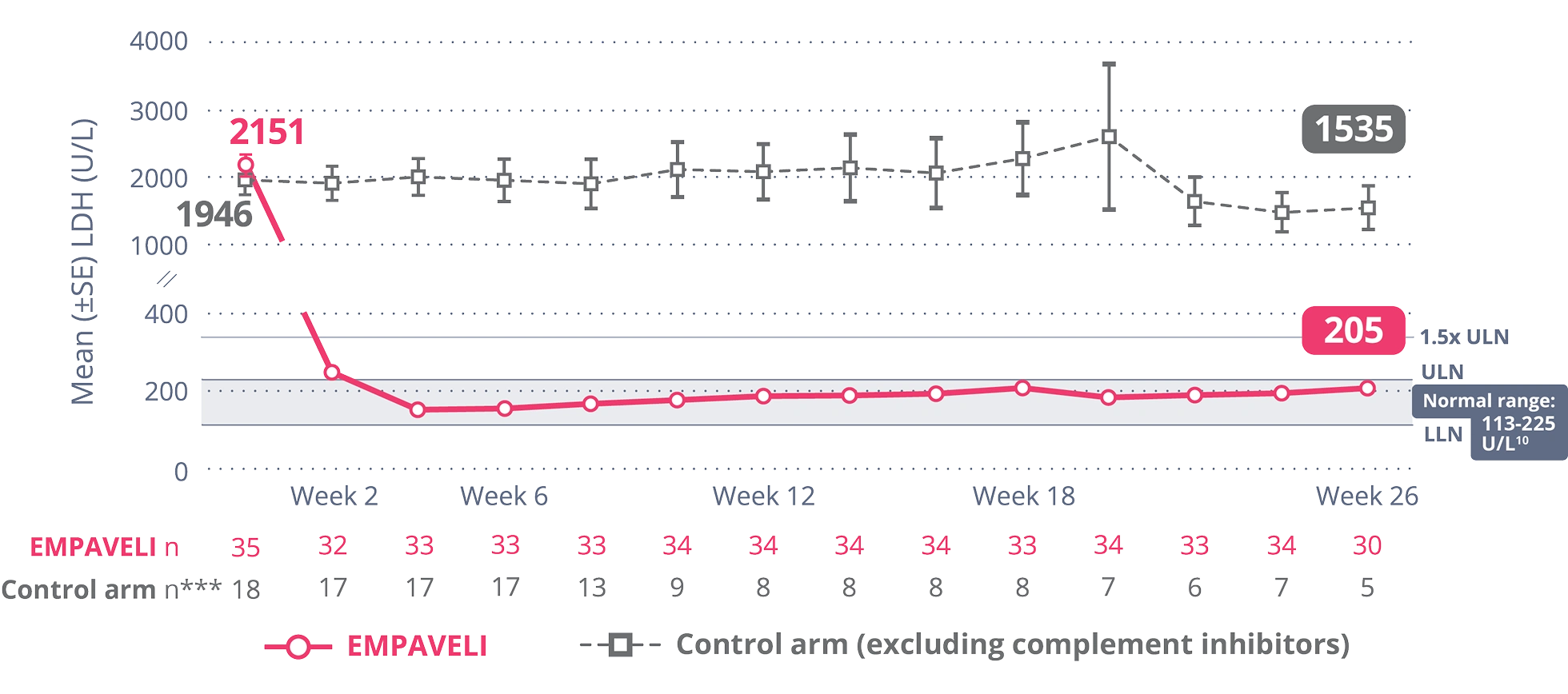
EMPAVELI reduced LDH to normal levels at Week 2, an effect that was sustained through Week 265,10
At Week 26,
change from baseline was -1870 U/L for patients taking EMPAVELI vs -400 U/L for patients in the control arm***
(P<0.0001) (95% CI, -2113.4, -827.3)1,†††
***Control arm only includes data on or before escape.
†††The post-baseline missing values (including the values after crossover from the control arm) are imputed using a multiple imputation method.
Most patients remained transfusion free through Week 26
with EMPAVELI1,10
Freedom from transfusion at Week 26 achieved in1:
Remaining transfusion free may have significant clinical benefits for patients.6
EMPAVELI normalized Hb and LDH levels in complement inhibitor–naïve
patients10
The data from these secondary analyses are descriptive in nature and collected for observation only. No formal comparisons can be drawn between the 2 arms.
Nearly 1/2 of patients achieved Hb normalization with EMPAVELI at Week 261,10,‡‡‡
normalization
was achieved in 46% of patients taking EMPAVELI (n=35)
in the control arm (excluding complement inhibitors)
(n=18)
Nearly 2/3 of patients achieved LDH normalization at Week 261,10,§§§
normalization
was achieved in 66% of patients taking EMPAVELI (n=35)
in the control arm (excluding complement inhibitors)
(n=18)
Both analyses were censored for transfusions.5
‡‡‡Hb normalization is defined as an Hb level at or above the lower limit of the gender-specific normal range, which is ≥12 g/dL to 16 g/dL for females and ≥13.6 g/dL to 18 g/dL for males.5,10
§§§LDH normalization is defined as an LDH level at or below the upper limit of normal range, which is 113 U/L to 226 U/L.5,10
AND PRINCE (COMPLEMENT INHIBITOR-NAÏVE) TRIALS5
Long-term sustained Hb levels for up to 3 years11,||||||,¶¶¶
Observed median values in Hb (g/dL) up to 3 years5
Patients who had a transfusion were not included in the Hb analysis for 60 days after the transfusion.12
Normal range of Hb: 12 g/dL to 16 g/dL for females, 13.6 g/dL to 18 g/dL for males.3
||||||Baseline: baseline laboratory value of patients upon initiation of EMPAVELI, regardless of when in the trials this occurred.12
¶¶¶Data reflect the subset of patients who received 1080 mg of EMPAVELI by subcutaneous infusion twice weekly or every 3 days.12
Long-term sustained LDH data for up to 3 years11,###,****
Observed median values in LDH (g/dL) up to 3 years3,5
LDH normalization is defined as 113 U/L to 226 U/L.3
###Baseline: baseline laboratory value of patients upon initiation of EMPAVELI, regardless of when in the trials this occurred.12
****Data reflect the subset of patients who received 1080 mg of EMPAVELI by subcutaneous infusion twice weekly or every 3 days.12

Individual patient experiences may vary.
ARC=absolute reticulocyte count; C5i=C5 inhibitor; EMP=EMPAVELI; FACIT=Functional Assessment of Chronic Illness Therapy; ITT=intent to treat; Hb=hemoglobin; LDH=lactate dehydrogenase; LLN=lower limit of normal; OLP=open-label period; PNH=paroxysmal nocturnal hemoglobinuria; RCP=randomized control period; SE=standard error; ULN=upper limit of normal.
References: 1. EMPAVELI [prescribing information]. Waltham, MA: Apellis Pharmaceuticals, Inc.; 2025. 2. Heo YA. Pegcetacoplan: a review in paroxysmal nocturnal haemoglobinuria. Drugs. 2022;82(18):1727-1735. doi:10.1007/s40265-022-01809-w 3. Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384(11):1028-1037. doi:10.1056/NEJMoa2029073 4. de Latour RP, Szer J, Weitz IC, et al. Pegcetacoplan versus eculizumab in patients with paroxysmal nocturnal haemoglobinuria (PEGASUS): 48-week follow-up of a randomised, open-label, phase 3, active-comparator, controlled trial. Lancet Haematol. 2022;9(9):e648-e659. doi:10.1016/S2352-3026(22)00210-1 5. Data on file. Apellis Pharmaceuticals, Inc., Waltham, MA. 6. Gao C, Li L, Chen B, et al. Clinical outcomes of transfusion-associated iron overload in patients with refractory chronic anemia. Patient Prefer Adherence. 2014;8:513-517. doi:10.2147/PPA.S56238 7. Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2024;390(11)(suppl1):1060. doi:10.1056/NEJMx240003 8. Cella D, Sarda SP, Hsieh R, et al. Changes in hemoglobin and clinical outcomes drive improvements in fatigue, quality of life, and physical function in patients with paroxysmal nocturnal hemoglobinuria: post hoc analyses from the phase III PEGASUS study. Ann Hematol. 2022;101(9):1905-1914. doi:10.1007/s00277-022-04887-8 9. Desai D, Fishman J, Zhang X. Rapid time to a clinically meaningful response in FACIT-Fatigue scores with pegcetacoplan in patients with paroxysmal nocturnal hemoglobinuria: a Kaplan-Meier analysis from the PEGASUS trial. Clin Lymphoma Myeloma Leuk. 2022;22(2):S306. doi.org/10.1016/S2152-2650(22)01399-4 10. Wong RSM, Navarro-Cabrera JR, Comia NS, et al. Pegcetacoplan controls hemolysis in complement inhibitor-naive patients with paroxysmal nocturnal hemoglobinuria. Blood Adv. 2023;7(11):2468-2478. doi:10.1182/bloodadvances.2022009129 11. Horneff R, Czech B, Yeh M, Surova E. Three years on: the role of pegcetacoplan in paroxysmal nocturnal hemoglobinuria (PNH) since its initial approval. Int J Mol Sci. 2024;25(16):8698. doi:10.3390/ijms25168698 12. de Castro C, Mulherin B, Patriquin CJ, et al. Efficacy and safety is maintained up to 3 years in adults with paroxysmal nocturnal hemoglobinuria receiving pegcetacoplan. Blood. 2023;142(suppl1):574-576.
IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS INFECTIONS CAUSED BY ENCAPSULATED BACTERIA
EMPAVELI, a complement inhibitor, increases the risk of serious infections, especially those caused by encapsulated bacteria, such as Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B. Life-threatening and fatal infections with encapsulated bacteria have occurred in patients treated with complement inhibitors. These infections may become rapidly life-threatening or fatal if not recognized and treated early.
- Complete or update vaccination for encapsulated bacteria at least 2 weeks prior to the first dose of EMPAVELI, unless the risks of delaying therapy with EMPAVELI outweigh the risks of developing a serious infection. Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for vaccinations against encapsulated bacteria in patients receiving a complement inhibitor.
- Patients receiving EMPAVELI are at increased risk for invasive disease caused by encapsulated bacteria, even if they develop antibodies following vaccination. Monitor patients for early signs and symptoms of serious infections and evaluate immediately if infection is suspected.
Because of the risk of serious infections caused by encapsulated bacteria, EMPAVELI is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the EMPAVELI REMS.
CONTRAINDICATIONS
- Hypersensitivity to pegcetacoplan or to any of the excipients
- For initiation in patients with unresolved serious infection caused by encapsulated bacteria including Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B
WARNINGS AND PRECAUTIONS
Serious Infections Caused by Encapsulated Bacteria
EMPAVELI, a complement inhibitor, increases a patient’s susceptibility to serious, life-threatening, or fatal infections caused by encapsulated bacteria including Streptococcus pneumoniae, Neisseria meningitidis (caused by any serogroup, including non-groupable strains), and Haemophilus influenzae type B. Life-threatening and fatal infections with encapsulated bacteria have occurred in both vaccinated and unvaccinated patients treated with complement inhibitors. The initiation of EMPAVELI treatment is contraindicated in patients with unresolved serious infection caused by encapsulated bacteria.
Complete or update vaccination against encapsulated bacteria at least 2 weeks prior to administration of the first dose of EMPAVELI, according to the most current ACIP recommendations for patients receiving a complement inhibitor. Revaccinate patients in accordance with ACIP recommendations considering the duration of therapy with EMPAVELI. Note that, ACIP recommends an administration schedule in patients receiving complement inhibitors that differs from the administration schedule in the vaccine prescribing information. If urgent EMPAVELI therapy is indicated in a patient who is not up to date with vaccines against encapsulated bacteria according to ACIP recommendations, provide the patient with antibacterial drug prophylaxis and administer these vaccines as soon as possible. The benefits and risks of treatment with EMPAVELI, as well as the benefits and risks of antibacterial drug prophylaxis in unvaccinated or vaccinated patients, must be considered against the known risks for serious infections caused by encapsulated bacteria.
Vaccination does not eliminate the risk of serious encapsulated bacterial infections, despite development of antibodies following vaccination. Closely monitor patients for early signs and symptoms of serious infection and evaluate patients immediately if an infection is suspected. Inform patients of these signs and symptoms and instruct patients to seek immediate medical care if these signs and symptoms occur. Promptly treat known infections. Serious infection may become rapidly life-threatening or fatal if not recognized and treated early. Consider interruption of EMPAVELI in patients who are undergoing treatment for serious infections.
EMPAVELI is available only through a restricted program under a REMS.
EMPAVELI REMS
EMPAVELI is available only through a restricted program under a REMS called EMPAVELI REMS, because of the risk of serious infections caused by encapsulated bacteria. Notable requirements of the EMPAVELI REMS include the following:
Under the EMPAVELI REMS, prescribers must enroll in the program. Prescribers must counsel patients about the risks, signs, and symptoms of serious infections caused by encapsulated bacteria, provide patients with the REMS educational materials, ensure patients are vaccinated against encapsulated bacteria at least 2 weeks prior to the first dose of EMPAVELI, prescribe antibacterial drug prophylaxis if patients’ vaccine status is not up to date and treatment must be started urgently, and provide instructions to always carry the Patient Safety Card both during treatment, as well as for 2 months following last dose of EMPAVELI. Pharmacies that dispense EMPAVELI must be certified in the EMPAVELI REMS and must verify prescribers are certified.
Further information is available at www.empavelirems.com or 1-888-343-7073.
Infusion-Related Reactions
Systemic hypersensitivity reactions (e.g., facial swelling, rash, urticaria, pyrexia) have occurred in patients treated with EMPAVELI, which may resolve after treatment with antihistamines. Cases of anaphylaxis leading to treatment discontinuation have been reported. If a severe hypersensitivity reaction (including anaphylaxis) occurs, discontinue EMPAVELI infusion immediately, institute appropriate treatment, per standard of care, and monitor until signs and symptoms are resolved.
Monitoring PNH Manifestations after Discontinuation of EMPAVELI
After discontinuing treatment with EMPAVELI, closely monitor for signs and symptoms of hemolysis, identified by elevated LDH levels along with sudden decrease in PNH clone size or hemoglobin, or reappearance of symptoms such as fatigue, hemoglobinuria, abdominal pain, dyspnea, major adverse vascular events (including thrombosis), dysphagia, or erectile dysfunction. Monitor any patient who discontinues EMPAVELI for at least 8 weeks to detect hemolysis and other reactions. If hemolysis, including elevated LDH, occurs after discontinuation of EMPAVELI, consider restarting treatment with EMPAVELI.
Interference with Laboratory Tests
There may be interference between silica reagents in coagulation panels and EMPAVELI that results in artificially prolonged activated partial thromboplastin time (aPTT); therefore, avoid the use of silica reagents in coagulation panels.
ADVERSE REACTIONS
Most common adverse reactions in patients with PNH (incidence ≥10%) were injection‑site reactions, infections, diarrhea, abdominal pain, respiratory tract infection, pain in extremity, hypokalemia, fatigue, viral infection, cough, arthralgia, dizziness, headache, and rash.
USE IN SPECIFIC POPULATIONS
Females of Reproductive Potential
EMPAVELI may cause embryo-fetal harm when administered to pregnant women. Pregnancy testing is recommended for females of reproductive potential prior to treatment with EMPAVELI. Advise female patients of reproductive potential to use effective contraception during treatment with EMPAVELI and for 40 days after the last dose.
INDICATION
EMPAVELI® (pegcetacoplan) is indicated for the treatment of adult patients with paroxysmal nocturnal hemoglobinuria (PNH).
Please see full Prescribing Information, including Boxed WARNING regarding serious infections caused by encapsulated bacteria, and Medication Guide.
- COLLAPSE
IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS INFECTIONS CAUSED BY ENCAPSULATED BACTERIA
EMPAVELI, a complement inhibitor, increases the risk of serious infections, especially those caused by encapsulated bacteria, such as Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B. Life-threatening and fatal infections with encapsulated bacteria have occurred in patients treated with complement inhibitors. These infections may become rapidly life-threatening or fatal if not recognized and treated early.
- Complete or update vaccination for encapsulated bacteria at least 2 weeks prior to the first dose of EMPAVELI, unless the risks of delaying therapy with EMPAVELI outweigh the risks of developing a serious infection. Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for vaccinations against encapsulated bacteria in patients receiving a complement inhibitor.
- Patients receiving EMPAVELI are at increased risk for invasive disease caused by encapsulated bacteria, even if they develop antibodies following vaccination. Monitor patients for early signs and symptoms of serious infections and evaluate immediately if infection is suspected.
Because of the risk of serious infections caused by encapsulated bacteria, EMPAVELI is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the EMPAVELI REMS.
CONTRAINDICATIONS
- Hypersensitivity to pegcetacoplan or to any of the excipients
- For initiation in patients with unresolved serious infection caused by encapsulated bacteria including Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B
WARNINGS AND PRECAUTIONS
Serious Infections Caused by Encapsulated Bacteria
EMPAVELI, a complement inhibitor, increases a patient’s susceptibility to serious, life-threatening, or fatal infections caused by encapsulated bacteria including Streptococcus pneumoniae, Neisseria meningitidis (caused by any serogroup, including non-groupable strains), and Haemophilus influenzae type B. Life-threatening and fatal infections with encapsulated bacteria have occurred in both vaccinated and unvaccinated patients treated with complement inhibitors. The initiation of EMPAVELI treatment is contraindicated in patients with unresolved serious infection caused by encapsulated bacteria.
Complete or update vaccination against encapsulated bacteria at least 2 weeks prior to administration of the first dose of EMPAVELI, according to the most current ACIP recommendations for patients receiving a complement inhibitor. Revaccinate patients in accordance with ACIP recommendations considering the duration of therapy with EMPAVELI. Note that, ACIP recommends an administration schedule in patients receiving complement inhibitors that differs from the administration schedule in the vaccine prescribing information. If urgent EMPAVELI therapy is indicated in a patient who is not up to date with vaccines against encapsulated bacteria according to ACIP recommendations, provide the patient with antibacterial drug prophylaxis and administer these vaccines as soon as possible. The benefits and risks of treatment with EMPAVELI, as well as the benefits and risks of antibacterial drug prophylaxis in unvaccinated or vaccinated patients, must be considered against the known risks for serious infections caused by encapsulated bacteria.
Vaccination does not eliminate the risk of serious encapsulated bacterial infections, despite development of antibodies following vaccination. Closely monitor patients for early signs and symptoms of serious infection and evaluate patients immediately if an infection is suspected. Inform patients of these signs and symptoms and instruct patients to seek immediate medical care if these signs and symptoms occur. Promptly treat known infections. Serious infection may become rapidly life-threatening or fatal if not recognized and treated early. Consider interruption of EMPAVELI in patients who are undergoing treatment for serious infections.
EMPAVELI is available only through a restricted program under a REMS.
EMPAVELI REMS
EMPAVELI is available only through a restricted program under a REMS called EMPAVELI REMS, because of the risk of serious infections caused by encapsulated bacteria. Notable requirements of the EMPAVELI REMS include the following:
Under the EMPAVELI REMS, prescribers must enroll in the program. Prescribers must counsel patients about the risks, signs, and symptoms of serious infections caused by encapsulated bacteria, provide patients with the REMS educational materials, ensure patients are vaccinated against encapsulated bacteria at least 2 weeks prior to the first dose of EMPAVELI, prescribe antibacterial drug prophylaxis if patients’ vaccine status is not up to date and treatment must be started urgently, and provide instructions to always carry the Patient Safety Card both during treatment, as well as for 2 months following last dose of EMPAVELI. Pharmacies that dispense EMPAVELI must be certified in the EMPAVELI REMS and must verify prescribers are certified.
Further information is available at www.empavelirems.com or 1-888-343-7073.
Infusion-Related Reactions
Systemic hypersensitivity reactions (e.g., facial swelling, rash, urticaria, pyrexia) have occurred in patients treated with EMPAVELI, which may resolve after treatment with antihistamines. Cases of anaphylaxis leading to treatment discontinuation have been reported. If a severe hypersensitivity reaction (including anaphylaxis) occurs, discontinue EMPAVELI infusion immediately, institute appropriate treatment, per standard of care, and monitor until signs and symptoms are resolved.
Monitoring PNH Manifestations after Discontinuation of EMPAVELI
After discontinuing treatment with EMPAVELI, closely monitor for signs and symptoms of hemolysis, identified by elevated LDH levels along with sudden decrease in PNH clone size or hemoglobin, or reappearance of symptoms such as fatigue, hemoglobinuria, abdominal pain, dyspnea, major adverse vascular events (including thrombosis), dysphagia, or erectile dysfunction. Monitor any patient who discontinues EMPAVELI for at least 8 weeks to detect hemolysis and other reactions. If hemolysis, including elevated LDH, occurs after discontinuation of EMPAVELI, consider restarting treatment with EMPAVELI.
Interference with Laboratory Tests
There may be interference between silica reagents in coagulation panels and EMPAVELI that results in artificially prolonged activated partial thromboplastin time (aPTT); therefore, avoid the use of silica reagents in coagulation panels.
ADVERSE REACTIONS
Most common adverse reactions in patients with PNH (incidence ≥10%) were injection‑site reactions, infections, diarrhea, abdominal pain, respiratory tract infection, pain in extremity, hypokalemia, fatigue, viral infection, cough, arthralgia, dizziness, headache, and rash.
USE IN SPECIFIC POPULATIONS
Females of Reproductive Potential
EMPAVELI may cause embryo-fetal harm when administered to pregnant women. Pregnancy testing is recommended for females of reproductive potential prior to treatment with EMPAVELI. Advise female patients of reproductive potential to use effective contraception during treatment with EMPAVELI and for 40 days after the last dose.
INDICATION
EMPAVELI® (pegcetacoplan) is indicated for the treatment of adult patients with paroxysmal nocturnal hemoglobinuria (PNH).
Please see full Prescribing Information, including Boxed WARNING regarding serious infections caused by encapsulated bacteria, and Medication Guide.

FOR US HEALTHCARE PROFESSIONALS ONLY
The site you are about to enter is intended for US healthcare professionals only.
Please confirm that you are a US healthcare professional.


Thank you for your message.
An Apellis representative will get in touch with you as soon as possible.


You are about to leave EMPAVELIhcp.com
You are about to leave EMPAVELIhcp.com to visit a third-party website. Apellis Pharamaceuticals, Inc. is not responsible for their content. We recommend reviewing the privacy policy and terms and conditions of any site you visit.
The PEGASUS study was published in The New England Journal of Medicine. Please note that there may be some differences between the information within this journal article and the EMPAVELI US Prescribing Information. Apellis Pharmaceuticals, Inc. funded the PEGASUS study and provided financial support for this publication. The authors’ affiliations and financial disclosures are available at NEJM.org.

You are about to leave EMPAVELIhcp.com
You are about to leave EMPAVELIhcp.com to visit a third-party website. Apellis Pharamaceuticals, Inc. is not responsible for their content. We recommend reviewing the privacy policy and terms and conditions of any site you visit.
The PRINCE study was published in Blood Advances. Please note that there may be some differences between the information within this journal article and the EMPAVELI US Prescribing Information. Apellis Pharmaceuticals, Inc. funded the PRINCE study and provided financial support for this publication. The authors’ affiliations and financial disclosures are available at ashpublications.org/bloodadvances.

