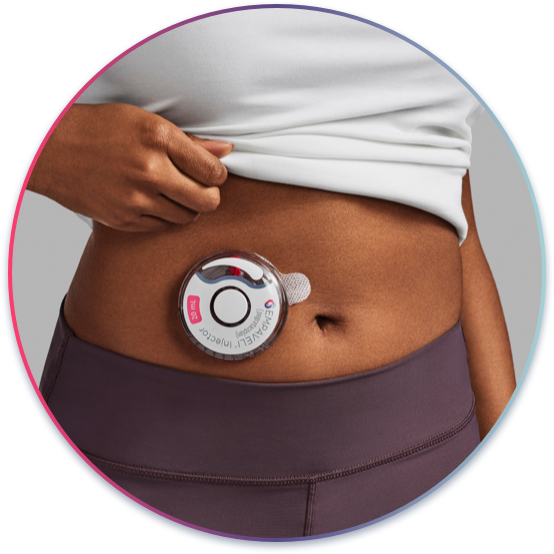
At-home self-administration that may fit into
a patient’s lifestyle
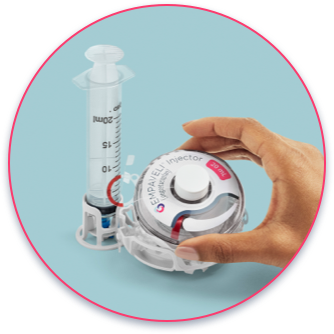

Each EMPAVELI injection should take approximately 30 to 60 minutes.
- Push button starts injection and pops up when injection is complete
- The needle is never seen
- Compact device with no tubing involved
- The gauge shows the injection progress
- No intravenous infusions
- No infusion center visits
- No need to plan around infusion appointments
Your patients should avoid intense physical activity and should not bump or knock the EMPAVELI Injector or button during the injection. Patients should keep their stomach totally dry.
Self-administration with EMPAVELI
EMPAVELI can be self-administered just under the skin. Patients can use1:
needle not visible to the patient
pump with a reservoir of at least 20 mL
The infusion will take approximately 30 to 60 minutes.1

needle is 7 mm, about the same
size as an insulin needle.
EMPAVELI is intended for use under the guidance of a healthcare professional. Patients should be trained on the self-administration process by a healthcare professional before they use EMPAVELI for the first time. Through the ApellisAssist program, Apellis Care Educators can provide patients with 1-on-1 self-administration training and support.
98% of patients reported they were confident self-administering EMPAVELI after training with an Apellis Care Educator.2,*
*Based on feedback from ~280 people with PNH after receiving self-administration training with an Apellis Care Educator. They rated their confidence with self-administration on a scale from 1 to 7. A score of ≥5 was considered “confident”. Data as of 02/20/2024.2
Patient compliance with
EMPAVELI self-administration is>97%2,†
†Compliance calculated by medical possession ratio of ~280 US patients on EMPAVELI. Data as of 12/2023.2
Vaccination requirements: What to know before prescribing EMPAVELI to your patients1
Vaccinate patients against encapsulated bacteria, including Streptococcus pneumoniae and Neisseria meningitidis (serogroups A, C, W, Y, and B), according to current ACIP recommendations at least 2 weeks prior to initiation of EMPAVELI therapy.
If urgent EMPAVELI therapy is indicated in a patient who is not up to date with these vaccines, according to ACIP recommendations, provide the patient with antibacterial drug prophylaxis and administer these vaccines as soon as possible.
The Risk Evaluation and Mitigation Strategy (REMS) program for EMPAVELI1
EMPAVELI is available only through a restricted program under a REMS called EMPAVELI REMS, because of the risk of serious infections caused by encapsulated bacteria. Notable requirements of the EMPAVELI REMS include the following:
Under the EMPAVELI REMS, prescribers must enroll in the program. Prescribers must counsel patients about the risks, signs, and symptoms of serious infections caused by encapsulated bacteria, provide patients with the REMS educational materials, ensure patients are vaccinated against encapsulated bacteria at least 2 weeks prior to the first dose of EMPAVELI, prescribe antibacterial drug prophylaxis if patients’ vaccine status is not up to date and treatment must be started urgently, and provide instructions to always carry the Patient Safety Card both during treatment, as well as for 2 months following the last dose of EMPAVELI. Pharmacies that dispense EMPAVELI must be certified in the EMPAVELI REMS and must verify prescribers are certified.
Further information is available at www.empavelirems.com or 1-888-343-7073.
Starting patients on EMPAVELI
Dosing: EMPAVELI is 1080 mg administered subcutaneously twice weekly.1
For the first 4 weeks, administer EMPAVELI twice weekly in addition to the patient’s current dose of eculizumab
After 4 weeks of concomitant therapy, discontinue eculizumab and start EMPAVELI monotherapy for the duration of therapy
Initiate EMPAVELI no more than 4 weeks after the last dose of ravulizumab
Concomitant treatment helps minimize the risk of hemolysis with abrupt treatment discontinuation.1
For LDH levels greater than 2X the ULN, adjust the dosing regimen to 1080 mg every 3 days
In the event of a dose increase, monitor LDH twice weekly for at least 4 weeks
Administer EMPAVELI as soon as possible after a missed dose. Resume the regular dosing schedule following administration of the missed dose
With EMPAVELI, the median effective half-life of elimination is 8.6 days in patients with PNH.1
How to self-administer EMPAVELI

“At first, I was a little worried I would mess up my self-administration, but with training, it didn't take long to get comfortable with it!”
Peg is a real patient who's taken EMPAVELI.
Individual patient experiences may vary.
ACIP=Advisory Committee on Immunization Practices; LDH=lactate dehydrogenase; PNH=paroxysmal nocturnal hemoglobinuria; REMS=Risk and Evaluation Mitigation Strategy; ULN=upper limit of normal.
References: 1. EMPAVELI [prescribing information]. Waltham, MA: Apellis Pharmaceuticals, Inc.; 2024. 2. Data on file. Apellis Pharmaceuticals, Inc., Waltham, MA.

