EMPAVELI demonstrated efficacy across
key measures of PNH disease activity1
3.84 g/dL
difference
with EMPAVELI
compared
to eculizumab1
(P<0.0001)
(95% Cl, 2.33-5.34)
Change from baseline in Hb levels at Week 16:
+2.37 g/dL with EMPAVELI vs –1.47 g/dL with eculizumab1
*Treatment effect estimates from a mixed-effects model are shown. The mixed-effects model contained the categorical effects of treatment, visit, treatment by visit interaction, and stratification factors (transfusion history and platelet count at screening), and the continuous covariate of baseline value.1
†Primary endpoint analysis of the PEGASUS data is censored for transfusions. Transfusions could confound the results, so data after the first transfusion for all patients were not included in the primary analysis. Posttransfusion data were omitted once they had a transfusion and their data were modeled out for the remainder of the 16-week RCP.2
Hb improvement with EMPAVELI was sustained through Week 483
Hb mean values observed (±SE, ITT, not censored for transfusions) from baseline to Week 483,‡
‡Primary analysis of the PEGASUS data is censored for transfusion, whereas observed data shown here do not censor posttransfusion data.
Transfusions could confound the results.
§Normal range of Hb: 12 to 16 g/dL for females, 13.6 to 18 g/dL for males.3

“I've been taking EMPAVELI for about a year now and my increased Hb has stayed consistent over time.”
Mike is a real patient who's taken EMPAVELI.
Individual patient experiences may vary.
Most patients with previous C5i experience (eculizumab) remained transfusion free through Week 16 with EMPAVELI1,2
Transfusion
avoidance was
achieved in
85%
of patients
taking EMPAVELI1,2
Transfusion avoidance at Week 16 met noninferiority2,‖
At Week 16, 85% of EMPAVELI patients (n=41) achieved transfusion avoidance, vs 15% of patients on eculizumab (n=39).1,2,¶
‖Noninferiority is a type of analysis that tests whether a new treatment is not worse than a comparator treatment by more than a specified margin.2
¶Sixty-three percent difference of mean values between EMPAVELI and eculizumab (95% CI, 48%-77%). Difference in percentages and 95% CI were based on the stratified Miettinen-Nurminen method.3
Remaining transfusion free may have significant clinical
benefits for patients.4
Change from baseline in ARC at Week 16 (censored for transfusion)
Noninferiority was met for the change from baseline in ARC level at Week 16.1
At Week 16, EMPAVELI patients experienced a change from baseline in ARC level of -136 x 109 cells/L, while eculizumab patients saw a change from baseline in ARC level of +28 x 109 cells/L.1,#
Mean baseline in ARC levels were 217.5 x 109 cells/L in the EMPAVELI treatment group and 216.2 x 109 cells/L in the eculizumab treatment group. Mean ARC at Week 16 for the EMPAVELI group was 77.65 x 109 cells/L, and 220.00 x 109 cells/L eculizumab group.3
#Difference of mean values was -164 between EMPAVELI (SE, 6.5; n=41) and eculizumab (SE, 11.9; n=39) (95% CI, -189.9 to -137.3).1
Change from baseline in LDH at Week 16 (censored for transfusion)
Noninferiority was not met for the change from baseline in LDH level at Week 16.3
The data are descriptive in nature and are for observation only. No formal comparisons can be drawn between the 2 arms.
The adjusted mean change from baseline in LDH level at Week 16 was -15 U/L in patients treated with EMPAVELI and -10 U/L in patients treated with eculizumab.3
Mean baseline LDH levels were 257.5 U/L in the EMPAVELI treatment group and 308.6 U/L in the eculizumab treatment group. At Week 16, mean LDH values were 189 U/L for patients treated with EMPAVELI and 183 U/L for patients treated with eculizumab.3
The normal range for LDH in this study was 113 U/L to 226 U/L.3
Adjusted mean change from baseline in FACIT-Fatigue scores at Week 16
Change from baseline in FACIT-Fatigue score was not tested for noninferiority, due to hierarchical testing.3
The data are descriptive in nature and are for observation only. No formal comparisons can be drawn between the 2 arms.
The adjusted mean change in FACIT-Fatigue score from baseline was +9.2 points in patients treated with EMPAVELI and -2.7 points in patients treated with eculizumab.2,**
Mean (±SE) FACIT-Fatigue scale score (0-52) over 16 weeks, uncensored for transfusion2
A ≥5-point difference is generally considered
clinically meaningful.5
**Primary analysis of the PEGASUS data is censored for transfusion. Transfusions could confound the results, so data after the first transfusion for all patients were not included in the primary analysis in order to truly assess the effect of each drug. Data posttransfusion were omitted once patients had a transfusion and their data were modeled out for the remainder of the 16-week RCP.2
Post hoc analyses from the PEGASUS study assessed fatigue5,6
Patients with greater improvements in hemoglobin showed a larger reduction in fatigue.5
Change in fatigue by hemoglobin at Week 16 (n=72)3,5
An Hb increase of ≥1 g/dL had an average fatigue
improvement of ~10 points.5
- Higher Hb levels indicated lower levels of fatigue3
- These post hoc analyses included patients receiving EMPAVELI and eculizumab5
The majority of patients achieved FACIT-Fatigue scores at the level of the general population with EMPAVELI.5
FACIT-Fatigue normalization was achieved when patient scores reached the general population mean of ≥43.6. FACIT-Fatigue normalization was achieved by 72% (23/32) of pegcetacoplan patients and 21% (6/29) of eculizumab patients.6
Many patients on EMPAVELI achieved Hb stabilization at Week 263
Hb stabilization
was achieved in
85.7%
of patients
taking EMPAVELI1
(P<0.0001)
(95% CI, 57%-89%)
At Week 26, 85.7% of EMPAVELI patients (n=35) achieved Hb stabilization, vs 0% of patients (n=18) in the control arm (excluding complement inhibitors).1,*
*Avoidance of a >1 g/dL decrease in hemoglobin levels from baseline in the absence of transfusions through Week 26 (yes/no). Patients who received a transfusion, escaped from control arm to the EMPAVELI treatment group, withdrew from trial, or were lost to follow-up were categorized as nonresponders. Cochran-Mantel-Haenszel test stratified by number of RBC transfusions within 12 months prior to screening (<4, ≥4).1
EMPAVELI achieved rapid and sustained LDH reductions through Week 261
At Week 26, change from baseline in LDH level was -1870 U/L for EMPAVELI-treated patients and -400 U/L for those in the control arm (P<0.0001) (95% CI, 2113.4-827.3).1
Reductions in LDH were sustained through Week 26 and mean LDH was normalized (below ULN)1,†
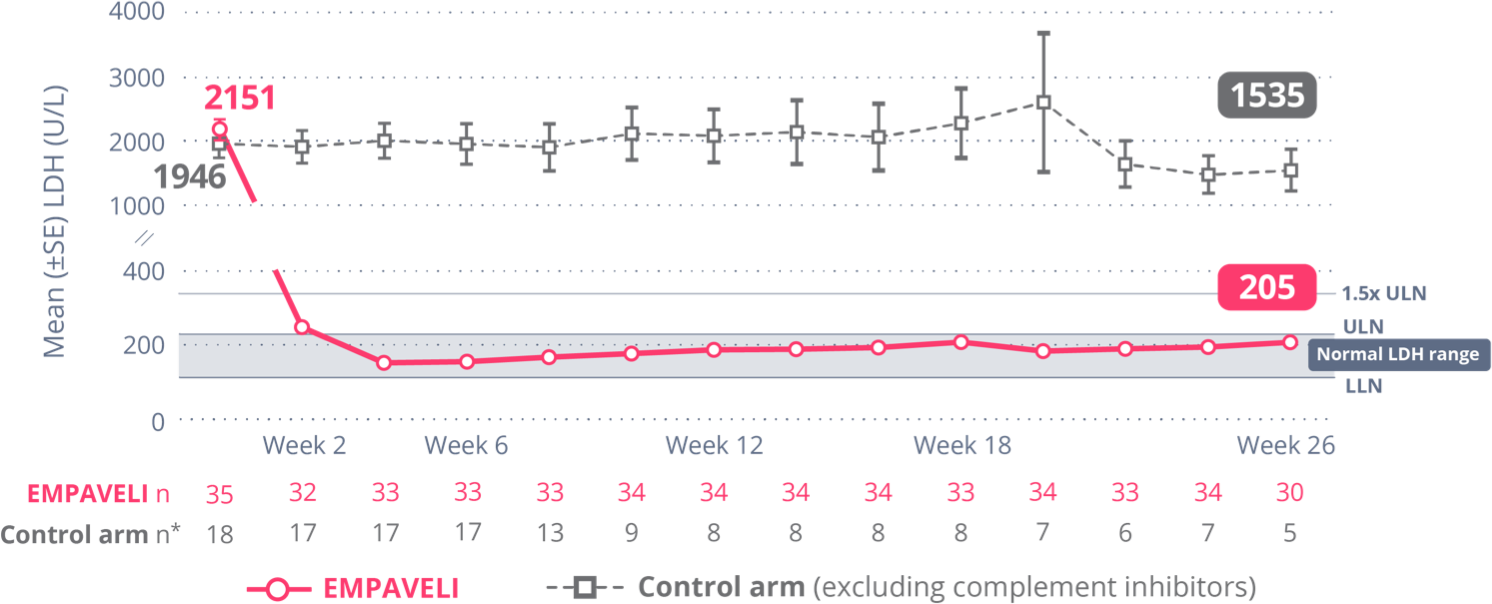
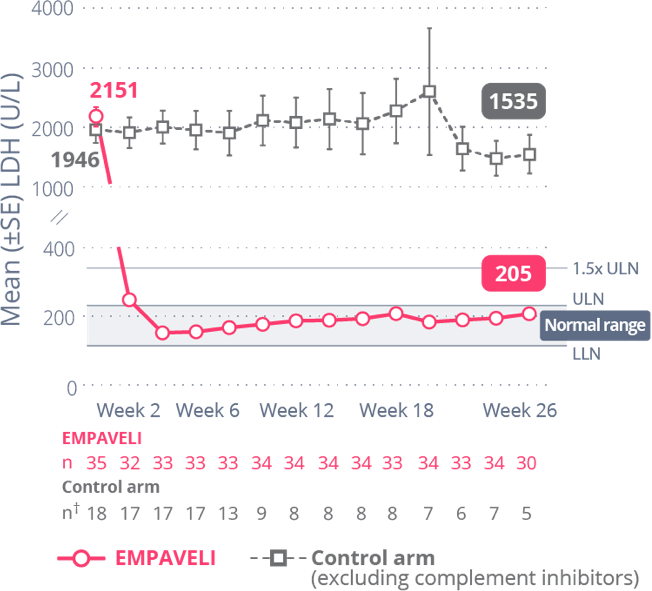
†Control arm only includes data on or before escape.
Most complement inhibitor–naïve patients remained transfusion free through Week 26 with EMPAVELI1
Freedom from
transfusion achieved in
91%
of patients
taking EMPAVELI1
(P<0.0001)
(95% CI, 56%-89%)
At Week 26, 91% of EMPAVELI patients (n=35) achieved transfusion avoidance, vs 6% of patients (n=18) in the control arm (excluding complement inhibitors).1
Remaining transfusion free may have significant clinical benefits for patients.4
ARC=absolute reticulocyte count; C5i=complement component 5 inhibitor; FACIT=Functional Assessment of Chronic Illness Therapy; ecu=eculizumab; EMP=EMPAVELI; Hb=hemoglobin; ITT=intent to treat; LDH=lactate dehydrogenase; LLN=lower limit of normal; OLP=open-label period; PNH=paroxysmal nocturnal hemoglobinuria; RCP=randomized control period; RBC=red blood cell; SE=standard error; ULN=upper limit of normal.
References: 1. EMPAVELI [prescribing information]. Waltham, MA: Apellis Pharmaceuticals, Inc.; 2024. 2. Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384:1028-1037. 3. Data on file. Apellis Pharmaceuticals, Inc., Waltham, MA. 4. Gao C, Li L, Chen B, et al. Clinical outcomes of transfusion-associated iron overload in patients with refractory chronic anemia. Patient Prefer Adherence. 2014;8:513-517. 5. Cella D, Sarda SP, Hsieh R, et al. Changes in hemoglobin and clinical outcomes drive improvements in fatigue, quality of life, and physical function in patients with paroxysmal nocturnal hemoglobinuria: post hoc analyses from the phase III PEGASUS study. Ann Hematol. 2022;101(9):1905-1914. 6. Desai D, Fishman J, Zhang X. Rapid time to a clinically meaningful response in FACIT-Fatigue scores with pegcetacoplan in patients with paroxysmal nocturnal hemoglobinuria: a Kaplan–Meier analysis from the PEGASUS trial. Clin Lymphoma Myeloma Leuk. 2022;22(2):S306. doi.org/10.1016/S2152-2650(22)01399-4

